In the first part, we focused on the dual role of IFNγ in the TME, now we analyze some cells that produce it instead.
Numerous subpopulations of immune cells produce IFNγ in the tumor microenvironment (TME). For a clearer and less verbose discussion, we will deal with only some cell types: such as some subtypes of T cells, Natural Killer (NK) cells and B cells. For those who would like to know more about this topic it is possible to consult the paper in which all other categories of immune cells of interest are listed.
Although IFNγ has pleiotropic effects, IFNγ production by immune cells is generally antitumorigenic rather than protumorigenic. However, IFNγ activity can be modified by other cytotoxic cytokines present in the tumor microenvironment (TME).
Now lets analyze some types of IFNγ-producing cells:
- Effector T cells
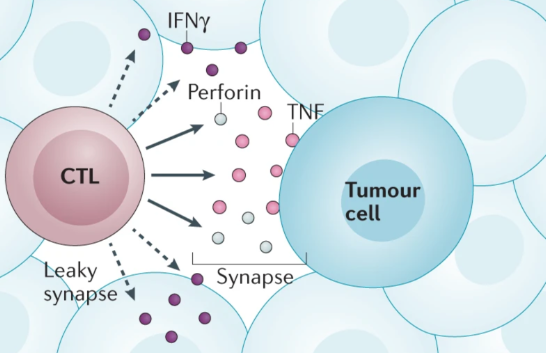
The CD8+ cytotoxic T lymphocytes (CTLs) are known producers of IFNγ and are critical for antitumor activity. In addition to expressing IFNγ they also produce other molecules such as perforin, granzymes, tumor necrosis factor (TNF) and interleukin-2 (IL-2).
When the T cell is activated, IFNγ release is stimulated so that the IFNv signal reaches both the target cell and the near cells. This mode of IFNγ secretion is different from that of other cytotoxic molecules such as TNF and perforin, which concentrate only at the immunological synapse, the contact zone between T cell and target cell, to mediate cancer cell killing in this case.
The researchers’ findings illustrate the importance of IFNγ production by CD8+ cytotoxic T cells in mediating both widespread and long-lasting beneficial effects of tumor cell killing and pro-inflammatory effects in the TME.
- “TH1” T cells
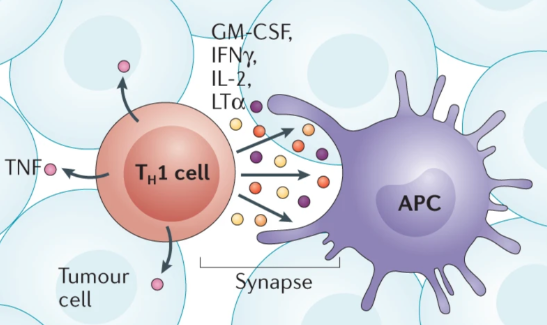
The IFNγ is also the characteristic cytokine of TH1 cells that also produce TNF and IL-2.
The release of IFNv and IL-2 is unidirectional and results in survival signals to specific immune cells, the APCs. In contrast, in TME, TNF is released from TH1 cells in a multidirectional manner to promote activation of specific immune cells, the macrophages. The importance of TH1 cells in TME was demonstrated in a mouse lung carcinoma model in which switching from a TME rich in TH2 cells, another type of T cell, to a TME dominated by TH1 cells promoted tumor elimination with combinatorial immunotherapies, which consists in using multiple drugs.
- NK Cells
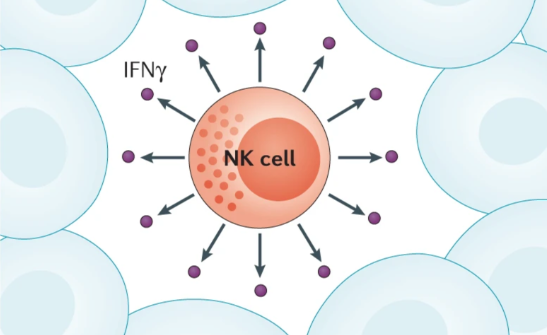
The NK cells are cytotoxic cells of the immune system that form the first line of defense against tumor growth.
Data collected on these cells suggest that their anti-tumorigenic functions are activated precisely by IFNγ.
NK cells have inhibitory receptors that keep them in a nonactivated state and activating receptors that instead activate the NK cell, which can then carry out its cytotoxic action, thus eliminating the target cells.
This cell type recognizes certain targets such as tumor cells and mediates cytotoxic effects through the production of IFNγ; in addition, the presence of NK cells within the tumor microenvironment is linked with a better prognosis in cancer patients. The presence of NK cells could be used in diagnostics, as suggested by Jongmi Lee and her collaborators, who published a paper in 2017 regarding a possible diagnostic test measuring the activity of NK cells in the blood for IFNv production in patients with gastric cancer. Such a method could become another noninvasive way to monitor disease progression.
- B cells
In addition to the ability to produce antibodies, B cells have antibody-independent functions through cytokine secretion. A subset of B cells produces IFNv during the early stages of bacterial infection, similarly to NK cells, grazid by expression of the receptor for IFNγ (IFNGR). However, the role of this subgroup of B cells in TME and how IFNγ is released are still unknown.
Today there are many other key questions that deserve further investigation, for example, which IFNγ-producing cells are most important for antitumor effects?, which IFNγ-expressing cells in the TME mediate resistance to immunotherapy? and how IFNγ can be introduced to make an “hot” tumor in a “cold” one?, that consists in turning tumors that do not see the presence of immune cells and cytokines in the TME into tumors with these elements within them.
Future studies should use innovative approaches to distinguish the proinflammatory from anti-inflammatory effects of IFNγ to design better therapies that affect its anti-tumor capabilities and prevent immune evasion.