Nowadays new cancer therapies see the engagement of our immune system to fight this disease.
For example, monoclonal antibodies represent a type of treatment to suppress cancer cell activity and alert the immune system; they can block the activation of different mechanisms within the cancer cell, such as uncontrolled proliferation.
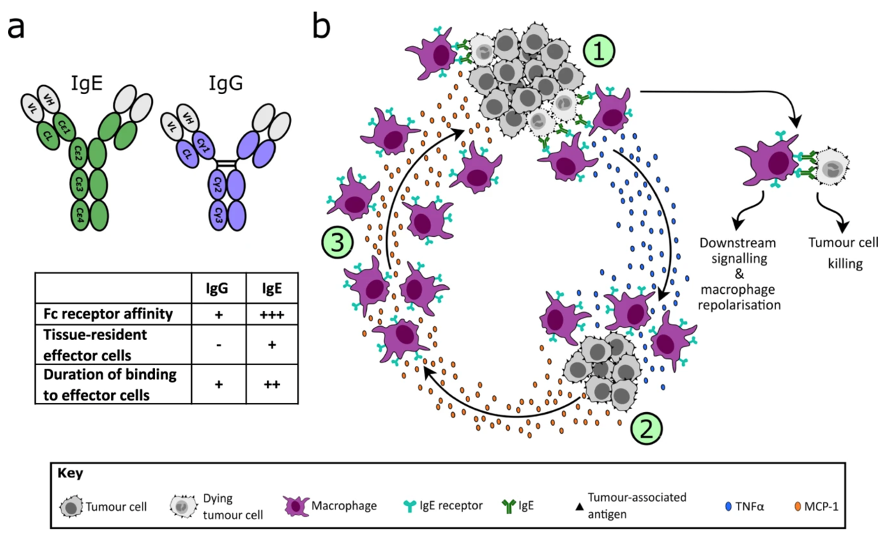
Today the monoclonal antibodies approved for cancer therapies belong to the most common type of antibody in the blood, IgG; however, researchers have also ventured into the use of IgE, another type of antibody, which offers the possibility of increasing the efficiency of the cells of the immune system responsible to eliminate abnormal cells, in this case the cancer cells.
Concerning this topic has been the focus of the research activity of James Spicer and collaborators. Their primary goal is to explore the safety and tolerability of MOv18 IgE, a chimeric antibody belonging to the first class of IgE.
This type of antibody is called chimeric because it is engineered in the laboratory and then reinfused into patients with tumors that express the relevant antigen, the antibody’s target.In the blood, circulating levels of IgE are lower than IgG, and IgE provides immune surveillance in tissues.
The mechanism by which therapeutic antibodies work is mediated in part by their binding to specific receptors, the Fc receptors that are present on the effector cells of the immune system.
The structure of Fc receptor for the IgE is different from that of IgG, and the affinity of IgE for its own receptors is higher compared with the affinity of IgG for their Fc receptors.
For this reason, drugs using IgE antibodies can mediate a more powerful immune response against cancer cells than IgG due to this higher affinity for Fc receptors.
Also through studies in animal models, the higher affinity of IgE binding to Fc receptors is thought to result in good therapeutic efficacy.
IgE induces cancer cell killing through mechanisms mediated by immune cells known as monocytes and macrophages.
The first step in testing this hypothesis is to determine whether IgE drugs can be safely administered to humans.
To verify their safety and to avoid possible undesirable effects, procedures were planned before the administration of MOv18 IgE:
- First, a skin test was performed with a solution with MOv18 IgE antibody inside, with the aim of selecting patients with a lower risk of allergic reactions. In fact, the most common reaction is urticaria. It could also cause activation of some immune cells, such as mast cells and basophils, and this could lead to an allergic reaction involving the whole body, known as an anaphylactic reaction. If the skin test is positive, pomphi and redness are present.
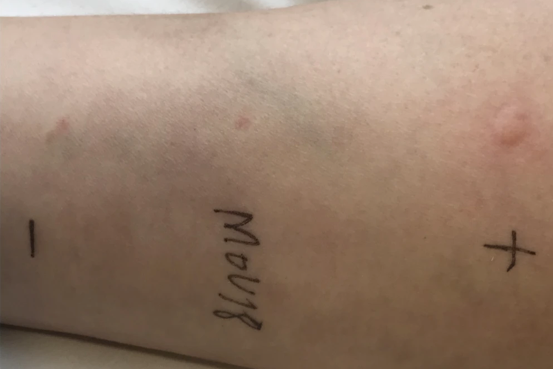
- Next a test was conducted to assess basophil activation in each patient’s blood using MOv18 IgE. This test is performed to avoid selecting patients who might have adverse reactions.
Researchers have observed preliminary evidence of the antitumor activity of MOv18 IgE in cases of high-grade serous ovarian cancer, with a decrease of the molecule CA125 and tumor reduction in a patient with chemotherapy-resistant disease. If this finding is confirmed in further clinical development, this antibody could become part of future therapies for the tumors expressing the MOv18 IgE’s target.
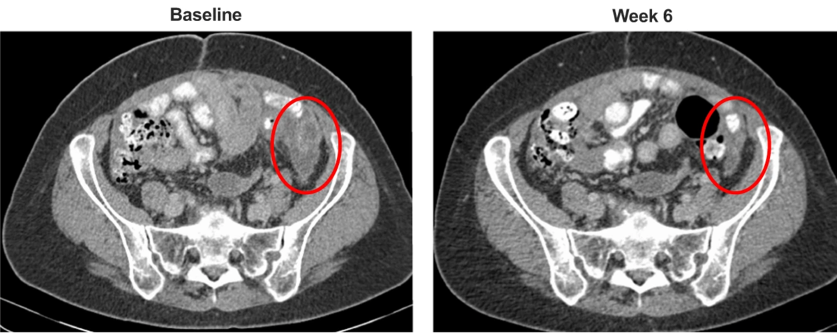
It was found that the antitumor activity and adverse events occurred at doses much lower than those typically observed for IgG antibodies, and the safety profile of MOv18 IgE is tolerable.
All of the results allow for the generation of a new class of IgE antibody-based anticancer therapies and to test the potential of IgE therapy for specific cancers.